The central theme of research in our lab is to understand how perception arises from the interplay between sensory encoding, environmental structure, and inference. We study how variations in the sensory filter – arising from optical blur, loss or degeneration of retinal or cortical neurons, or other changes in visual sensory transmission – tend to alter cortical representations, perceptual organization, and ultimately the way we recognize and interact with the world.
Our work is guided by the view that perception is an active, inferential process: the brain continually integrates incoming sensory signals with prior knowledge and environmental context to construct a coherent and actionable representations of the external world. When sensory information is degraded, the brain tends to compensate by relying more heavily on priors, predictions, and contextual cues at various stages of visual processing. Understanding these adaptive mechanisms helps reveal how perception remains robust in the face of uncertainty.
Our studies combine psychophysical measurement, eye tracking, fMRI, EEG, retinal imaging, and computational modeling across both normal and visually impaired populations, including those with macular degeneration, glaucoma, amblyopia and other retinal or cortical disorders. By linking changes in sensory encoding to cortical adaptation and perceptual behavior, we aim to deepen the scientific understanding of visual inference and neural plasticity while also translating this knowledge into improved diagnostic and rehabilitative strategies. Our research has been supported by the National Institutes of Health (NIH) and Research to Prevent Blindness (RPB).
Here are a few featured studies (click on any figure to access the related paper):
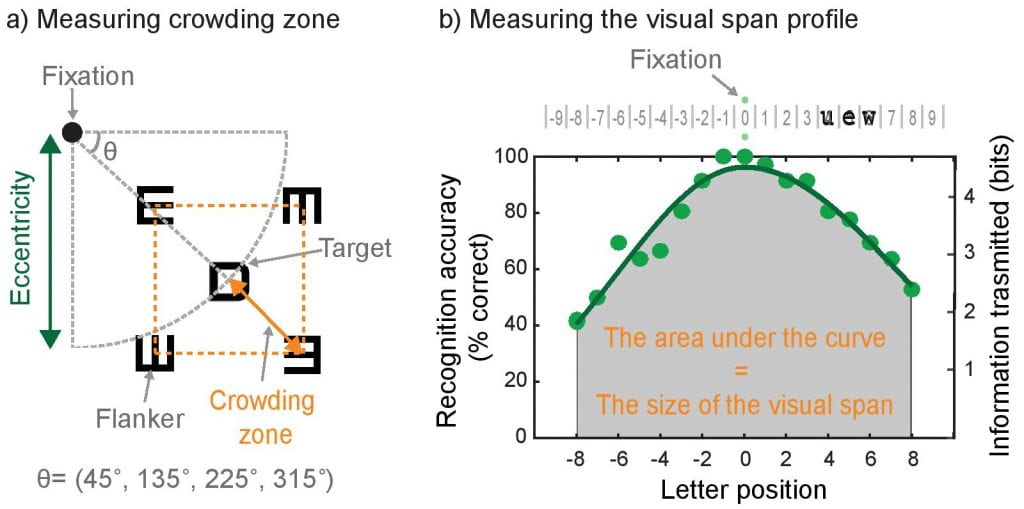
How RGC loss sets an upper bound on spatial summation and contributes to downstream functional deficits: This review highlights how glaucoma, traditionally viewed as a peripheral vision disease, also significantly impairs central visual function due to early loss of retinal ganglion cells (RGCs) in the macula. It synthesizes anatomical, psychophysical, and imaging evidence showing that glaucomatous damage compromises contrast sensitivity, increases visual crowding, and reduces the visual span—factors critical for everyday tasks like reading and face recognition.
How do early visual cortical areas process visual information under rod-dominant mesopic conditions? Mesopic (dim light) conditions are common in daily life, yet most vision research focuses on bright (photopic) settings. Retinal studies suggest that under mesopic conditions, receptive fields expand and surround inhibition weakens, enhancing light capture at the expense of spatial resolution. Here, we investigated whether cortical spatial summation and surround suppression reflect predictions based on retinal mechanisms. Our fMRI findings contrast with retinal electrophysiology and suggest that early visual cortex may employ distinct, perhaps compensatory, mechanisms in response to reduced retinal input under mesopic conditions.
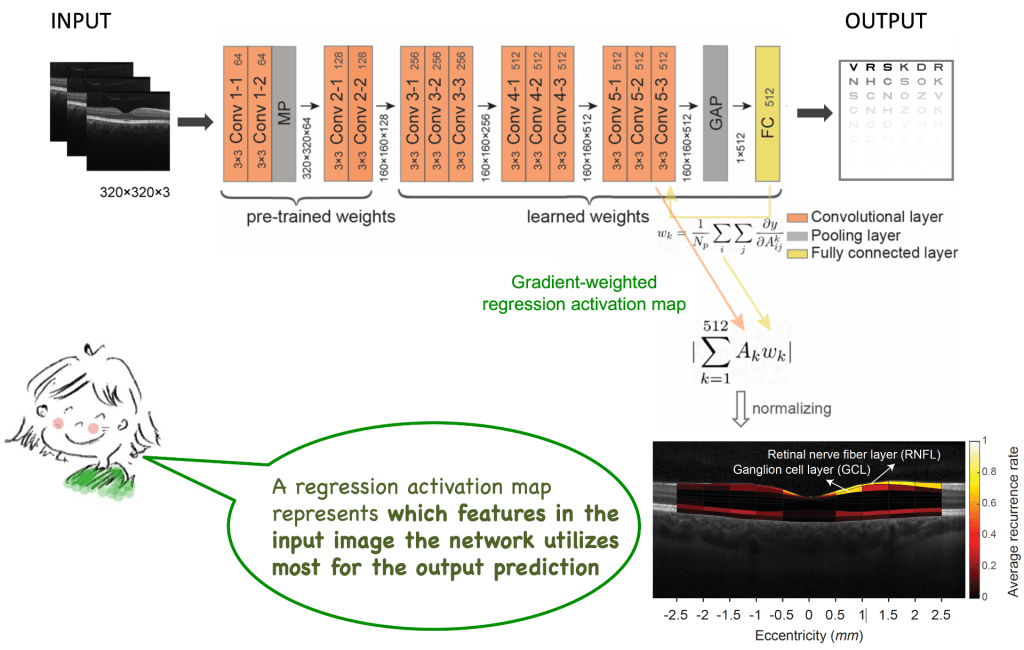
Identifying retinal layers closely linked to human contrast sensitivity and visual acuity: Using deep learning, we show that human contrast sensitivity (i.e., a building block of human pattern vision) can be reliably decoded from retinal structural (retinal imaging) data. The activation maps from the network help us identify and localize the exact retinal layers closely linked to human contrast sensitivity, i.e., the ganglion cell layers, where ganglion cell bodies and its dendritic structures are located.
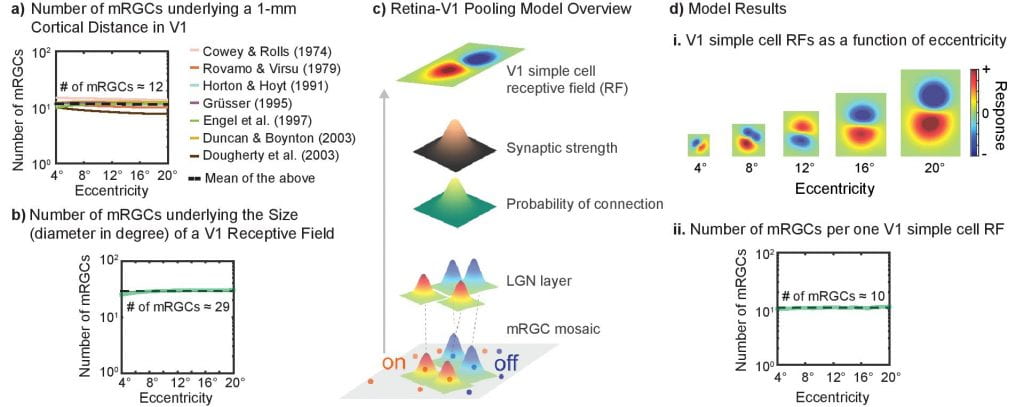
How computational constraints imposed by RGCs may set an upper bound on spatial integration such as crowding zone and Ricco’s area: Here we show that the variation in the sampling density of RGCs across the human retina is closely matched to the variation in the extent of spatial integration required for either luminance detection or object recognition. Our empirical data combined with the simulation results of computational models suggest that a fixed number of RGCs subserves spatial integration of visual input, independent of the visual-field location.
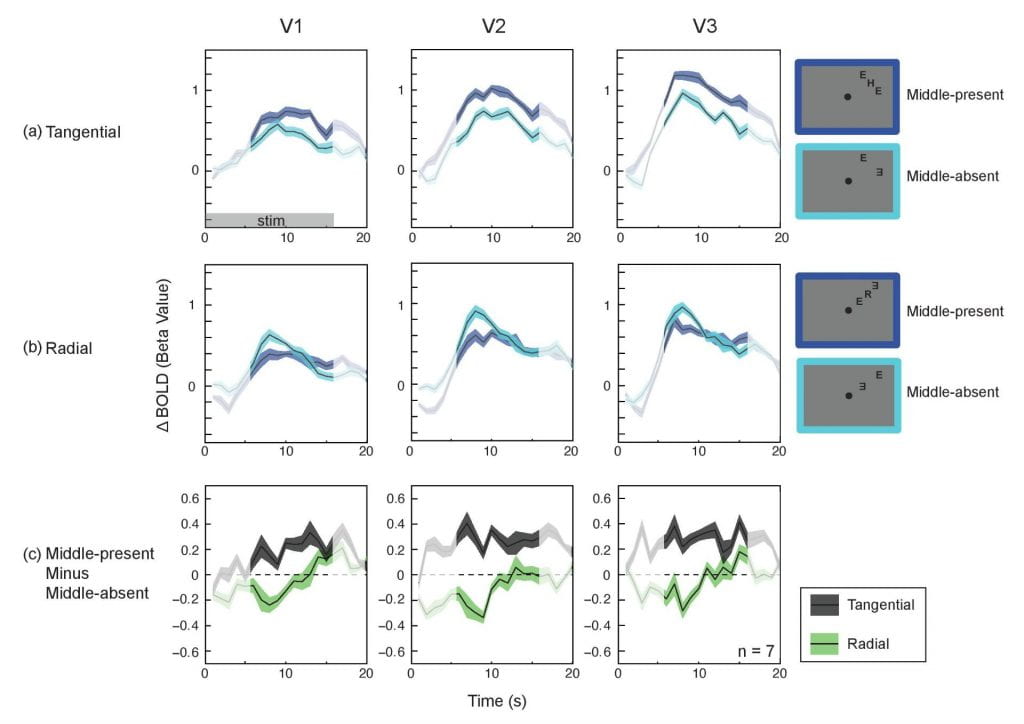
The signature radial-tangential anisotropy of crowding can be observed in V1, suggesting the locus of crowding can be as early as V1: Crowding, the inability to recognize an individual object in clutter, is considered a major impediment to object recognition in peripheral vision. Despite its significance, the cortical loci of crowding are not well understood. In particular, the role of the primary visual cortex (V1) remains unclear. Here we utilize a diagnostic feature of crowding to identify the earliest cortical locus of crowding.
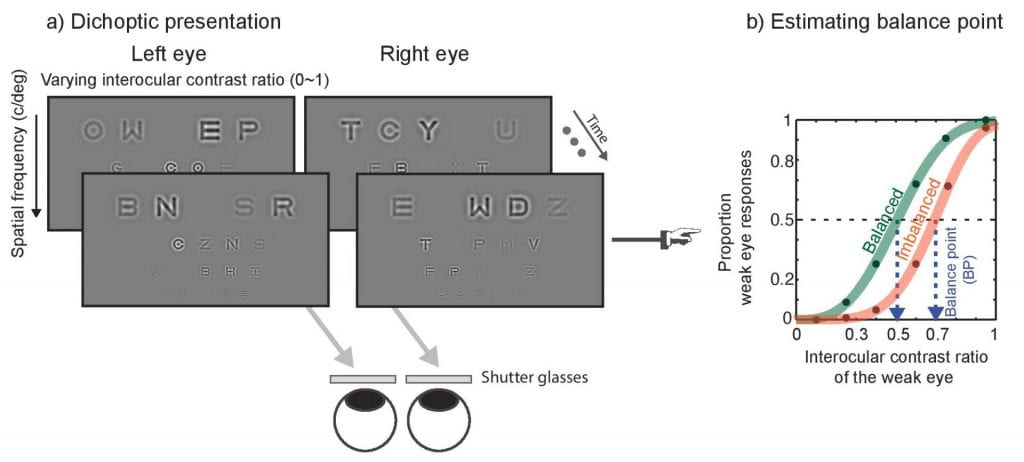
The role of spatial frequency in binocular interactions in amblyopia: Here we develop a novel dichotic letter chart to quantify binocular imbalance as a function of spatial frequency in those with abnormal binocular vision (e.g., amblyopia). As illustrated, two different sets of letter charts are presented to two different eyes using shutter glasses and the luminance contrast of letter systematically varies between the two eyes.
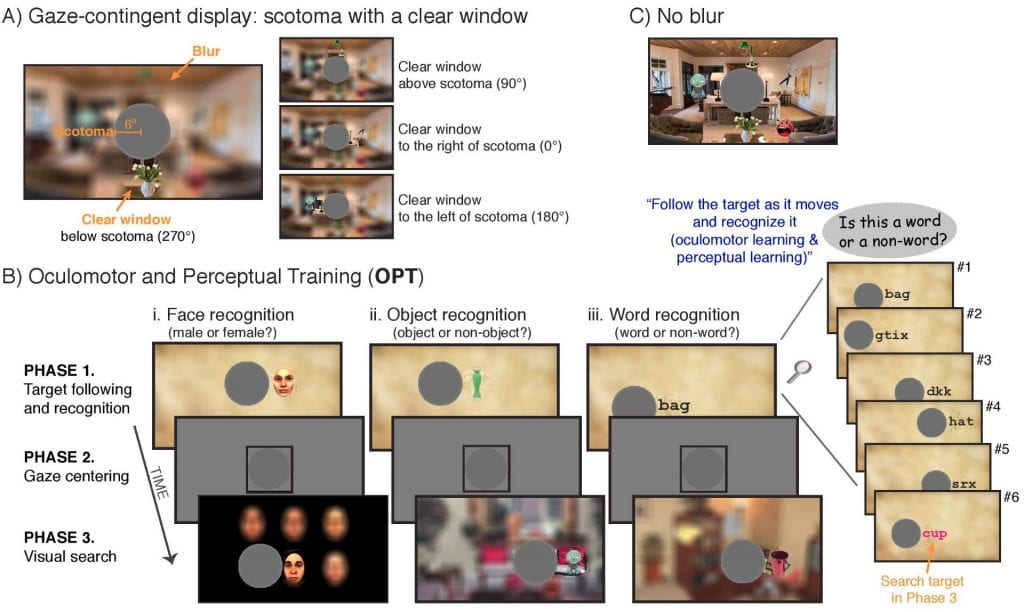
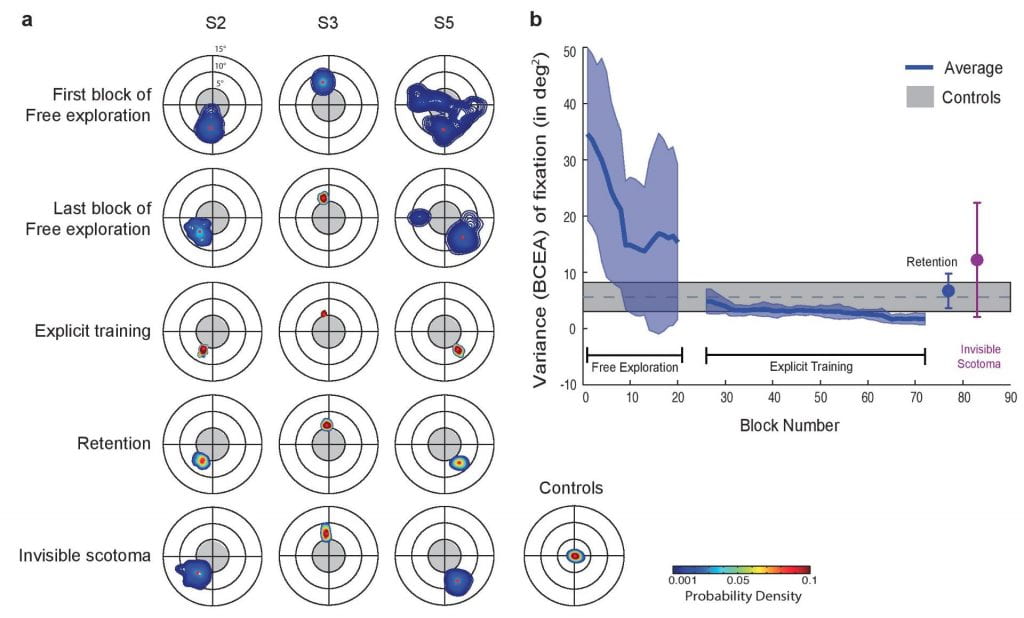
What kinds of perceptual and oculomotor changes occurs in previously unused retinal regions once they are adopted as a new fovea? People with a central scotoma often adopt an eccentric retinal location (Preferred Retinal Locus, PRL) for fixation. Here we develop a novel training paradigm as a model system to study the nature of the PRL formation and its impacts on visual function. The training paradigm is designed to effectively induce a PRL at any intended retinal location by integrating oculomotor control and pattern recognition.
How would the oculomotor system adjust to a loss of foveal vision (central scotoma)? Here we show that the oculomotor system can spontaneously and rapidly adopt a peripheral locus for fixation and can rereference saccades to this locus in normally sighted individuals whose central vision is blocked by an artificial scotoma.